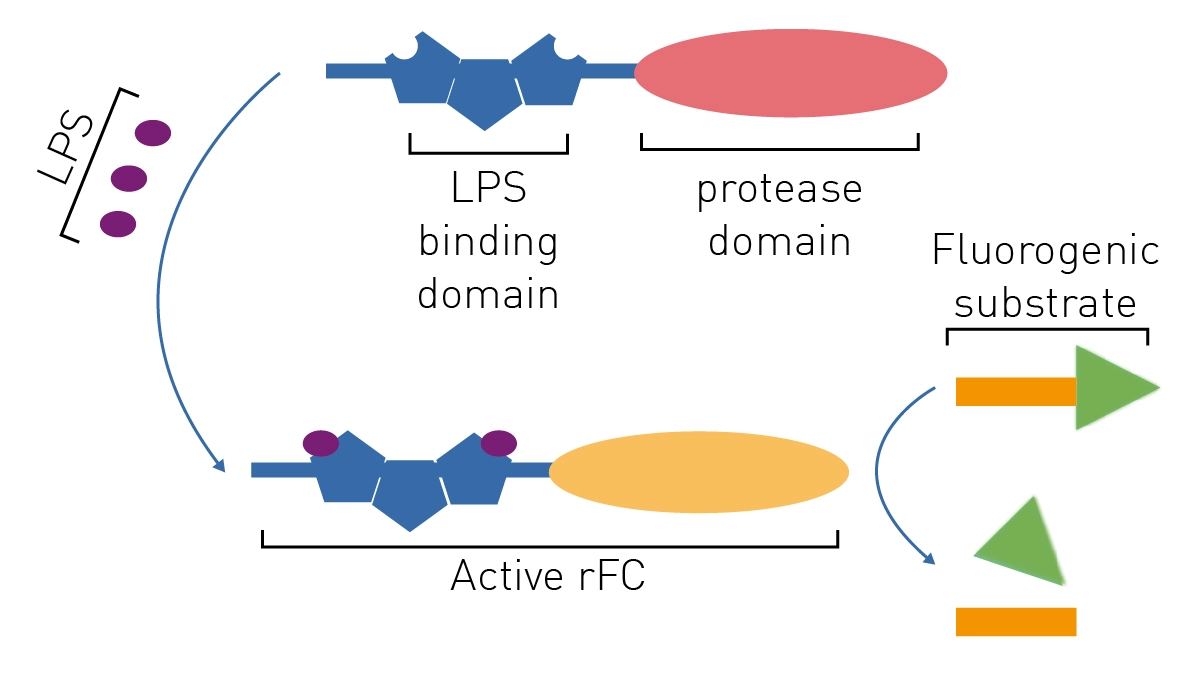
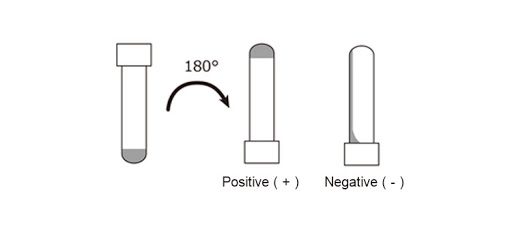
LAL test validation protocol flow-chart. The test was performed three... | Download Scientific Diagram
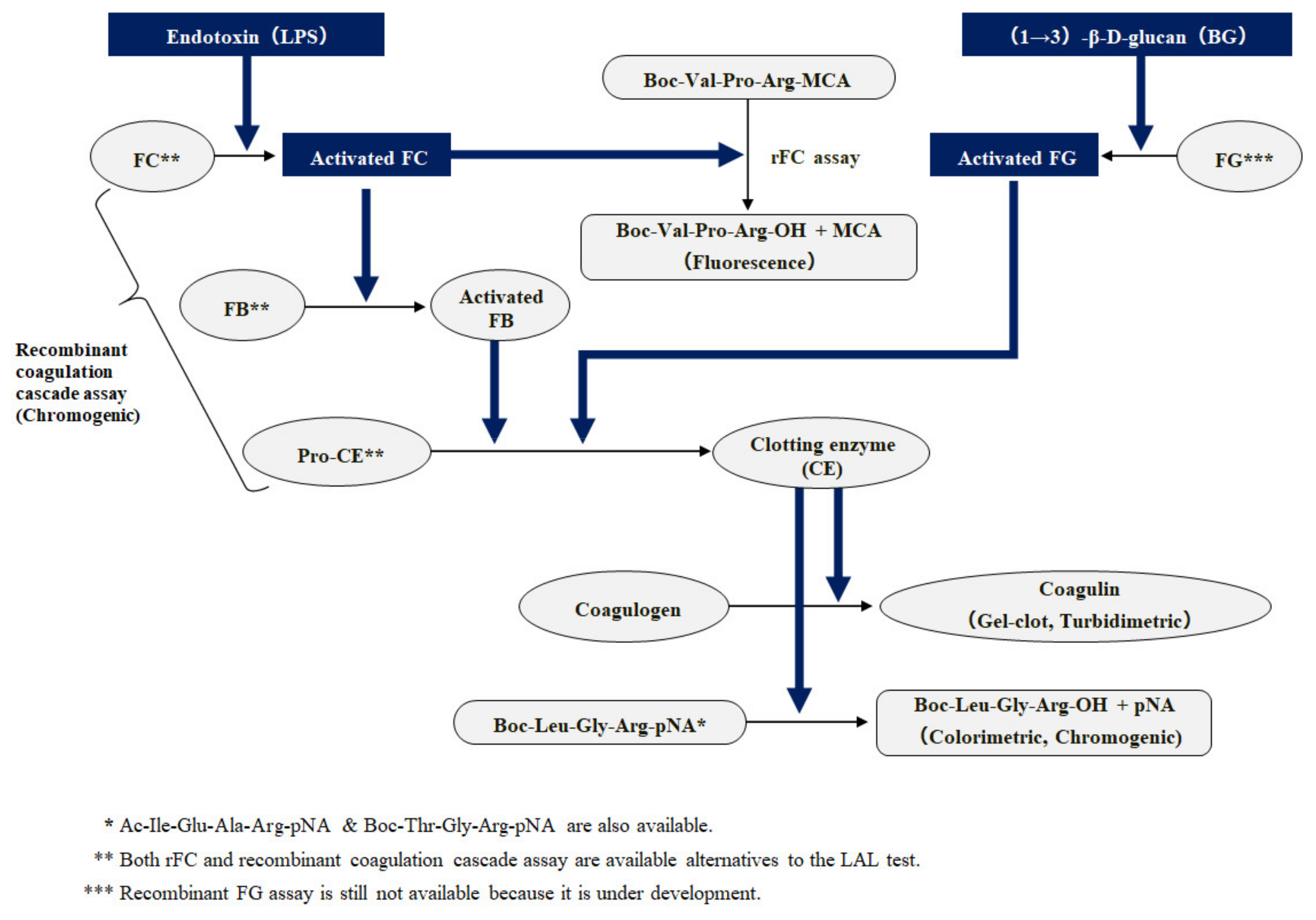
Biomedicines | Free Full-Text | Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and

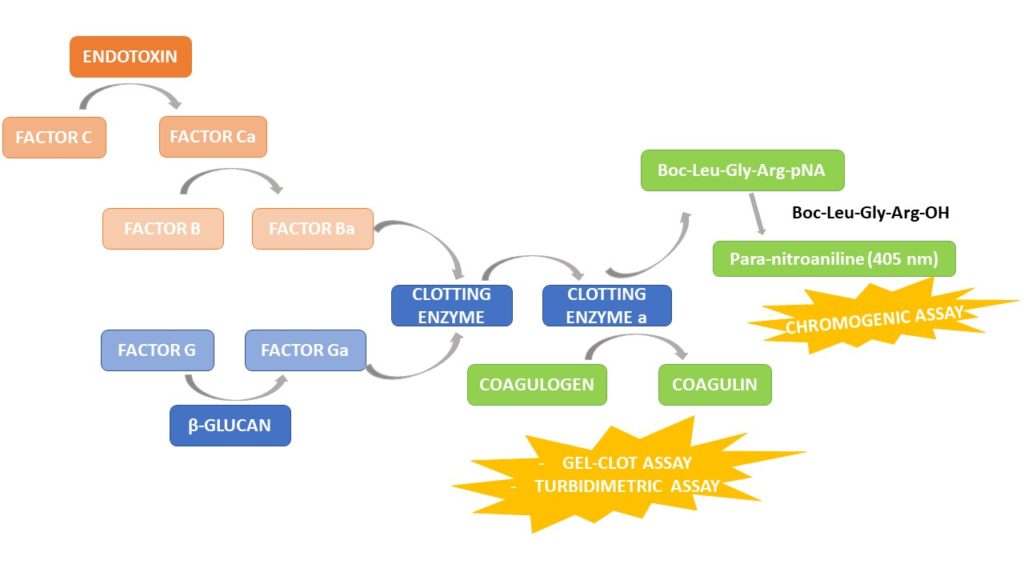
Test LAL, test dell'endotossina, reagente LAL, test del coagulo di gel, test del fattore C ricombinante, test dell'endotossina batterica, rilevamento dell'endotossina, produttori e fornitori di indicatori di endotossina - Tecnologia Bioendo