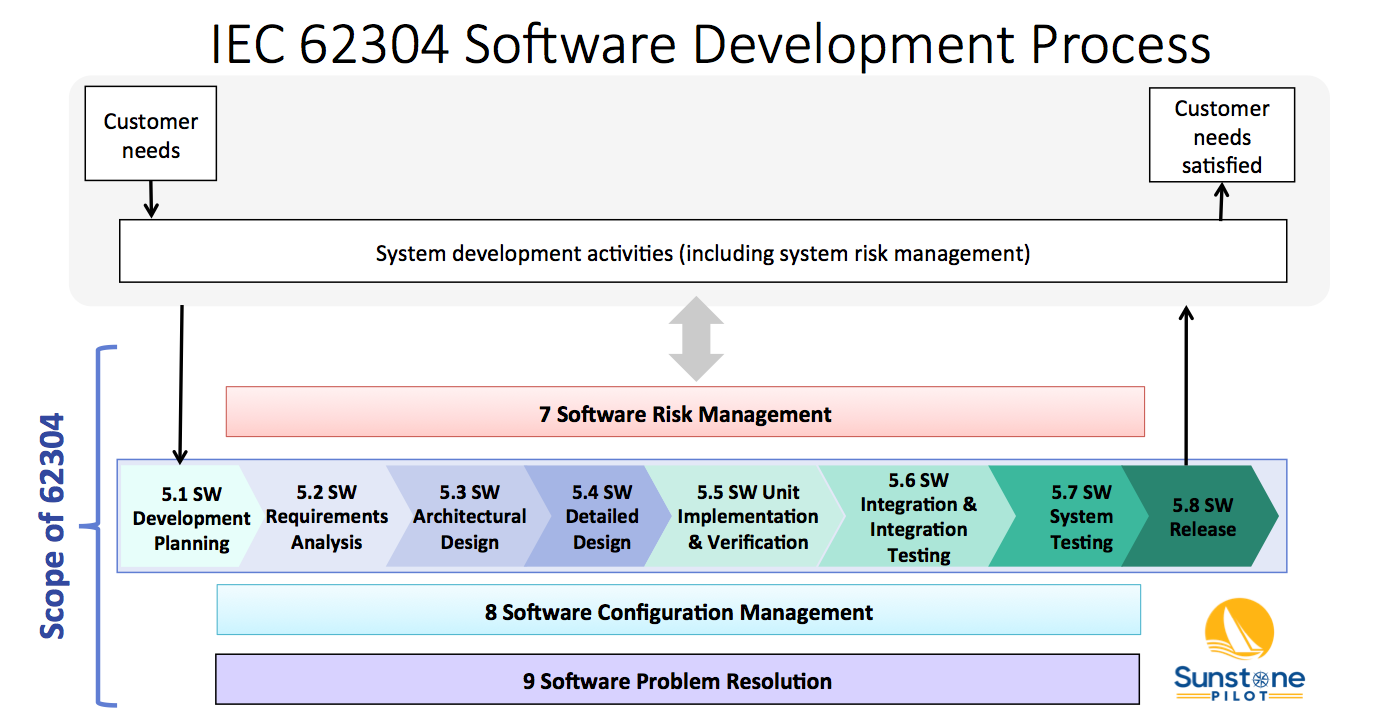
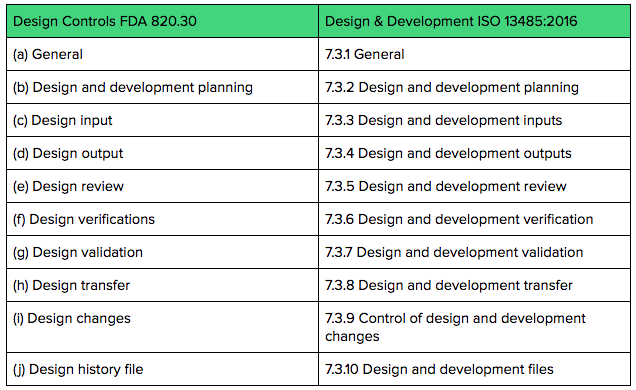
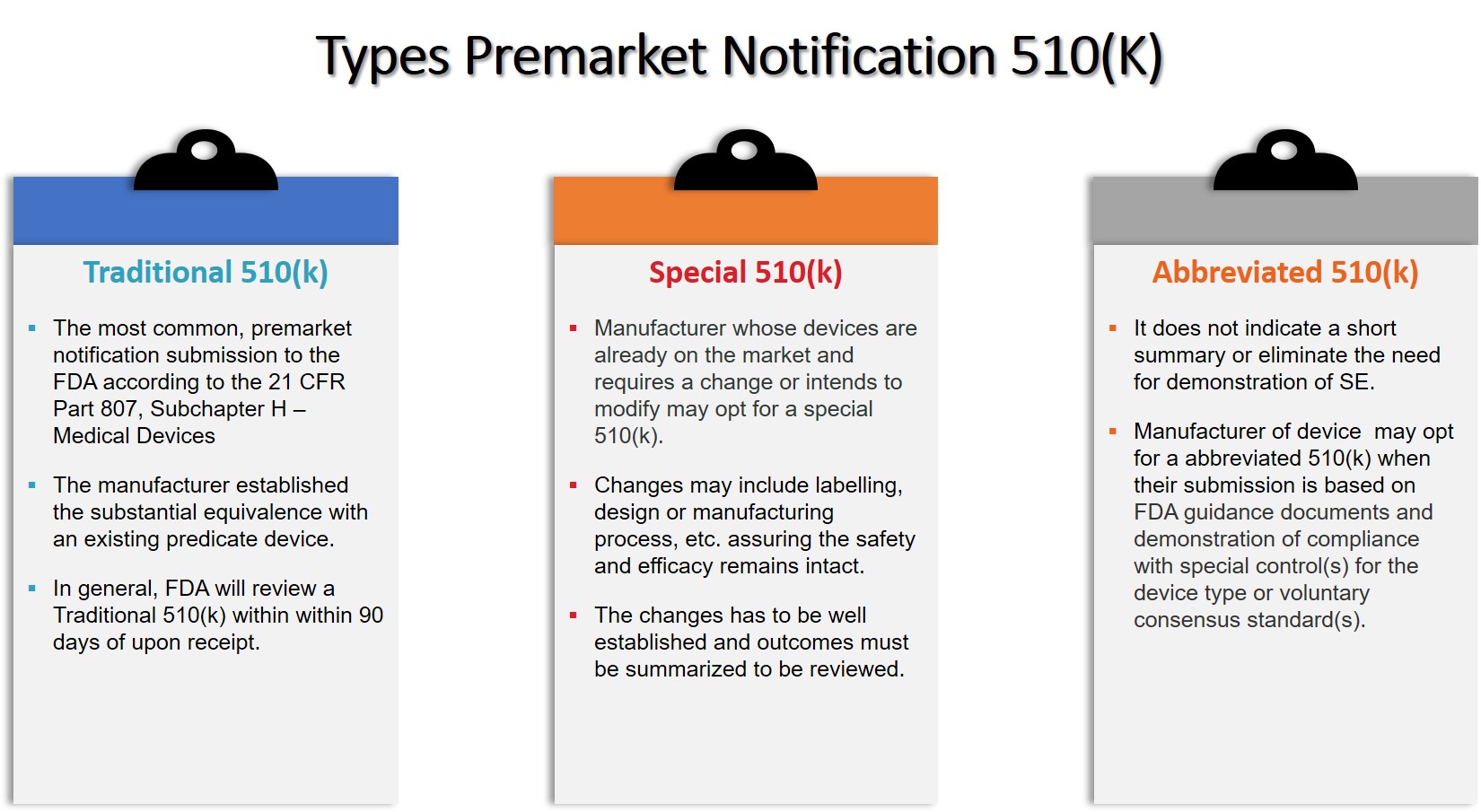
FDA Releases Two New Chapters for its Draft Guidance on the Preventive Controls for Human Food Rule: Food Allergen Program & Acidified Foods - InSilicoMinds provide AI, Modeling, & Simulation solutions for

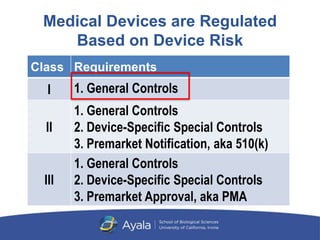
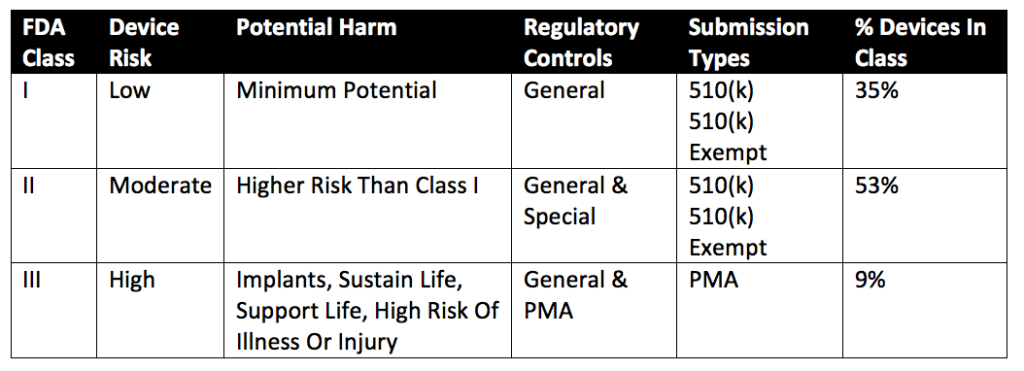
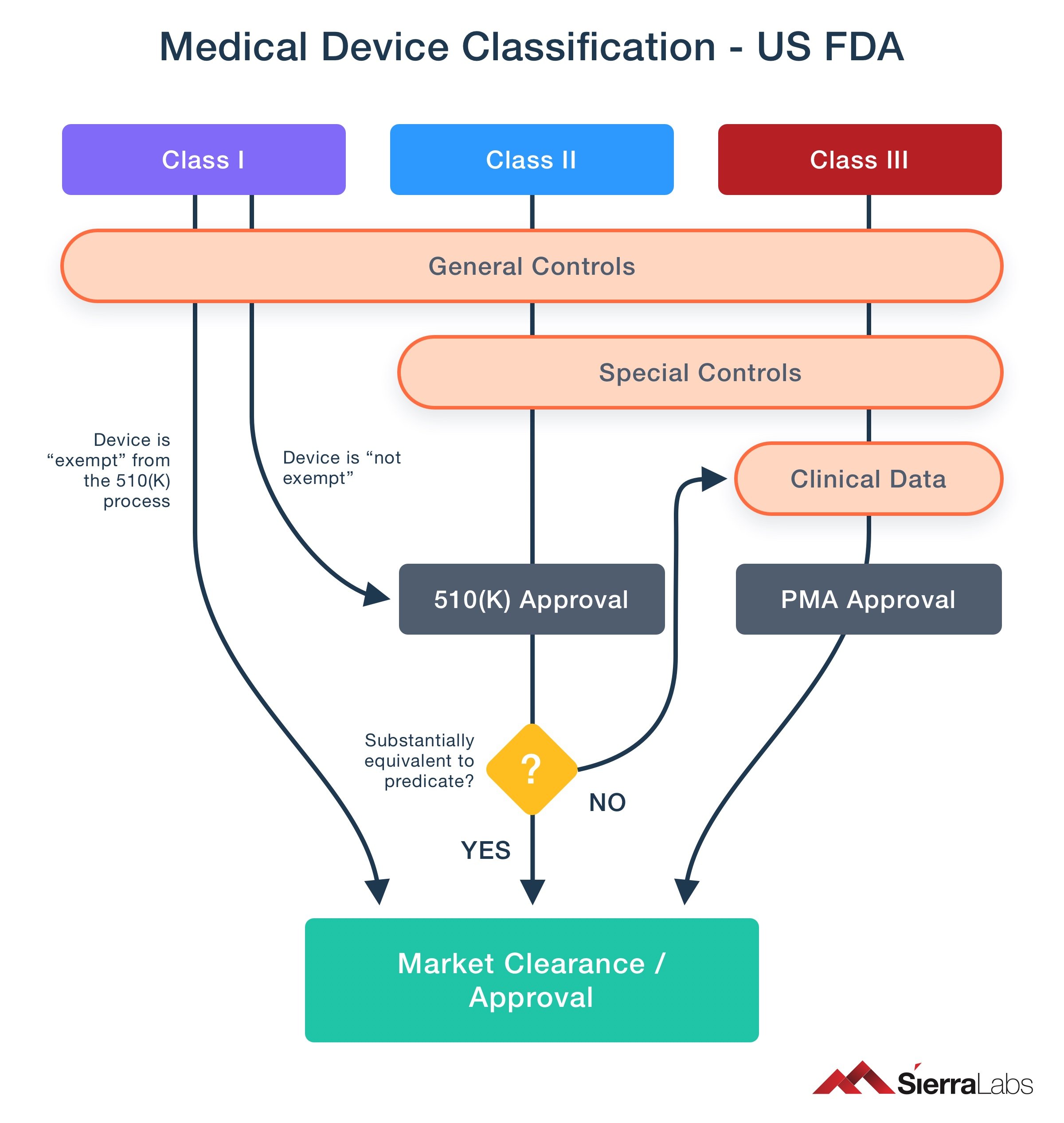
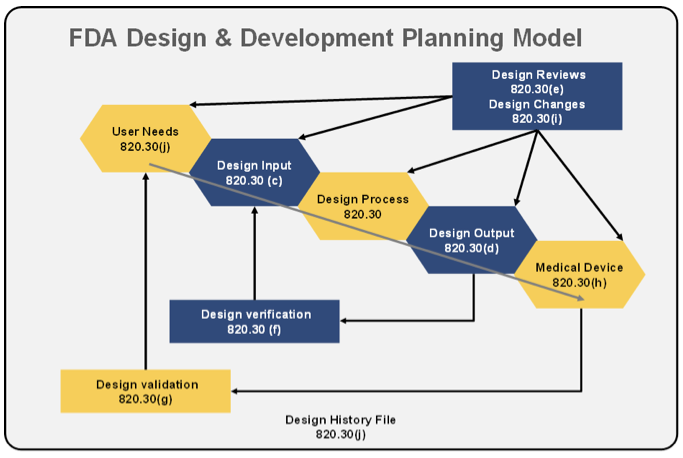
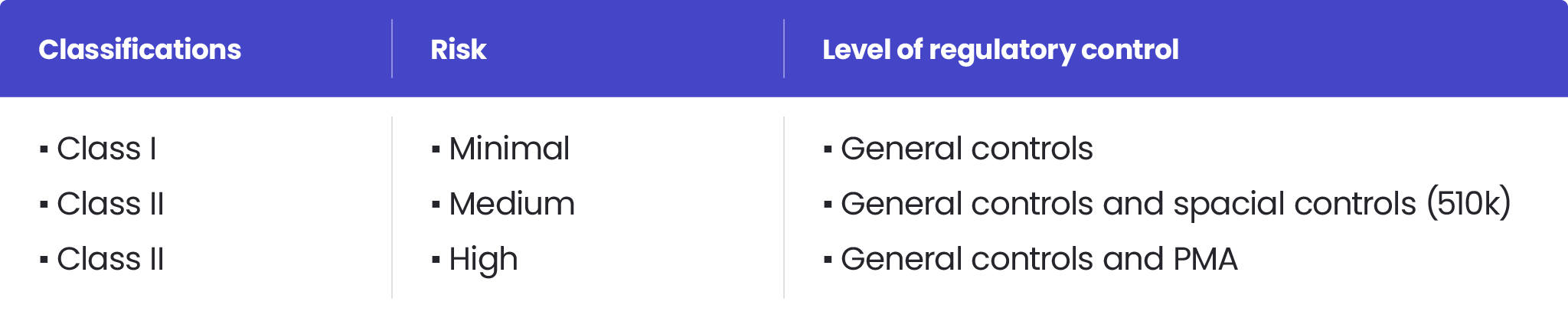
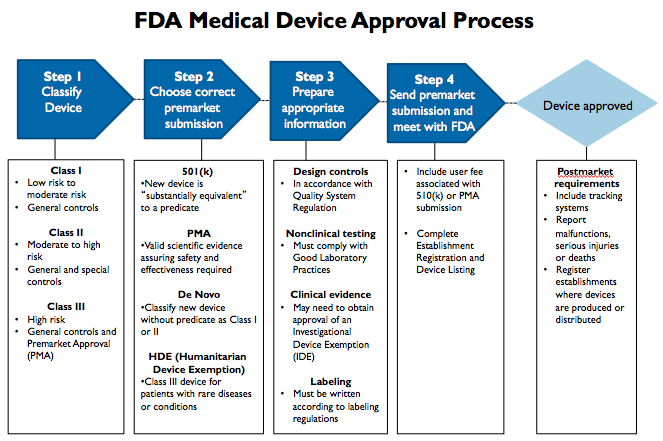
How the US FDA classifies Medical Devices | Risk management, Regulatory compliance, Statistical process control

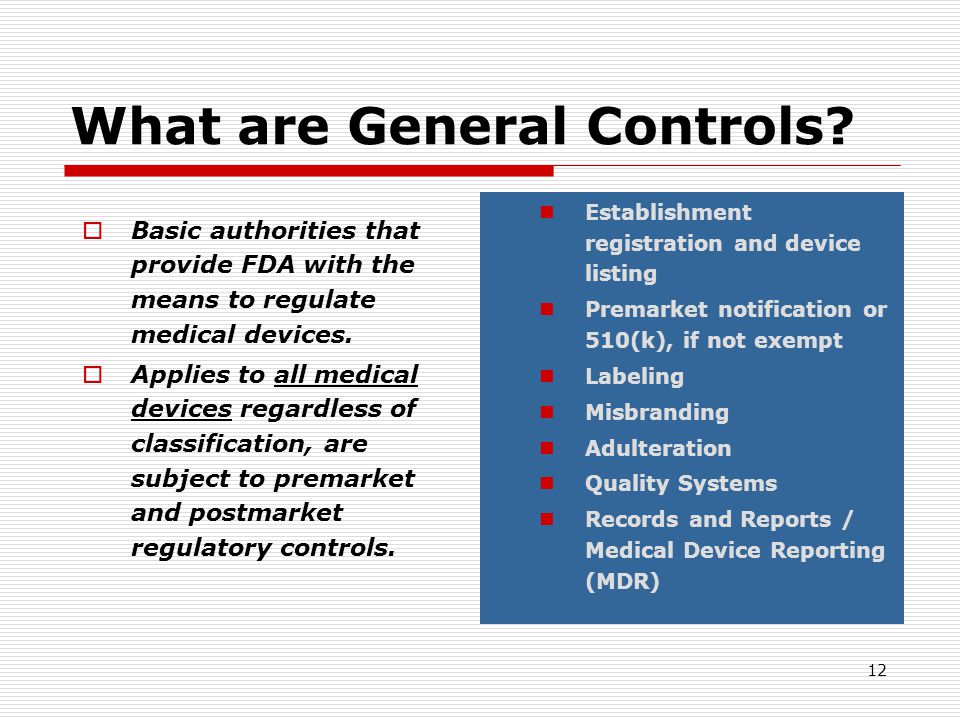


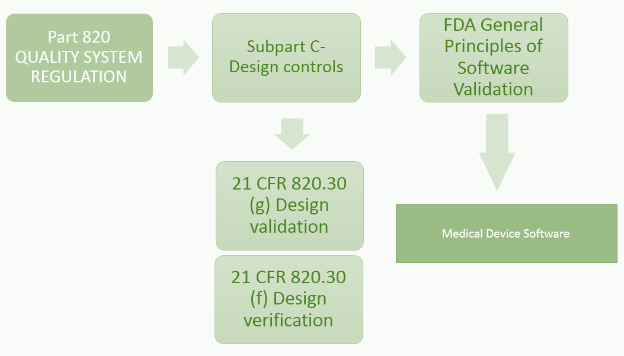
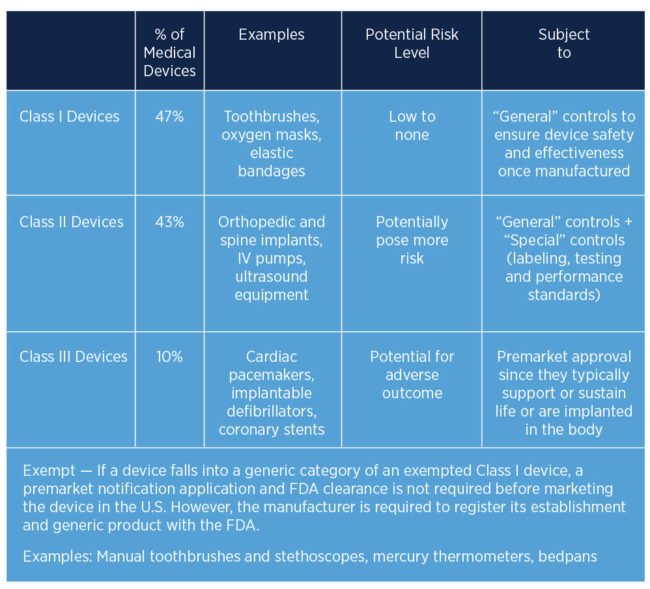
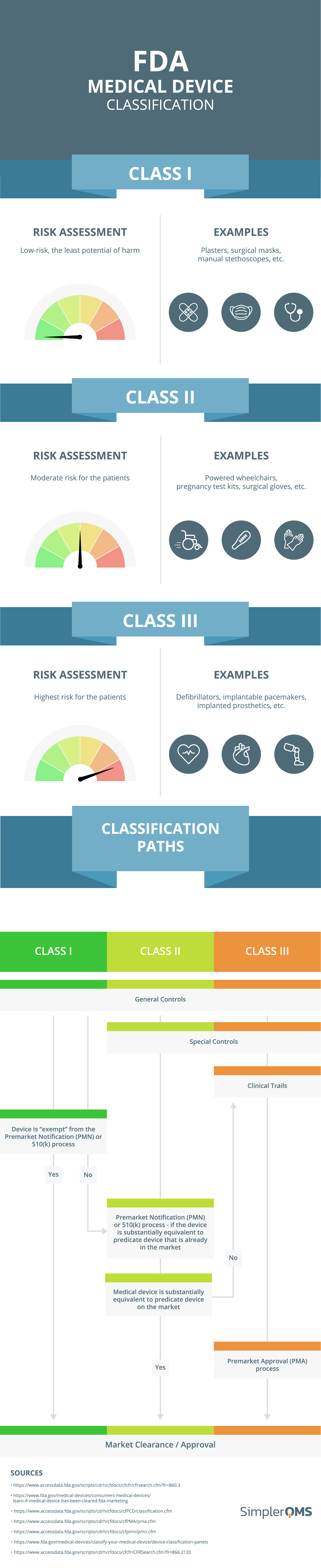
![PDF] FDA Regulation of Medical Devices | Semantic Scholar PDF] FDA Regulation of Medical Devices | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/24b77d41e78d641b874566fca26c1f2f2a89b548/8-Table1-1.png)